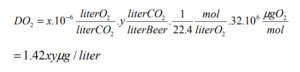Carbon Dioxide (CO2) purity is a very significant topic in modern brewing. Recently, many newer brewers have been picking up on the topic. Yet the topic is universal to all brewers, regardless of their hot side methods and we thought it might be a good idea to go back to the basics of the topic and discuss why CO2 purity can play such a large role in ushering in the initial stages of finished beer oxidation and staling.
One thing I think ALL parties can actually agree on is the subject of Cold Side Aeration (CSA). Lets take a minute to refresh our memory on what oxidation and staling is.
George Fix said it well here:
“The deleterious effects of oxygen uptake at any point in the brewing cycle is well documented. The only exception to this is the oxygen introduced at the start of the fermentation. It is true that there is a considerable variation among beer drinkers both with respect to their ability to detect oxidized flavors, and with respect to their acceptance of these notes. Yet the track record is clear in both amateur and commercial brewing: Consistently successful brewers are invariably the ones who operate low oxygen systems. For most of the twentieth century, attention was focused on oxidation occurring after the end of the fermentation-so-called cold side aeration (CSA). Concerns about CSA are well founded since there are a number of relevant mechanisms, all of which are destructive to beer flavor.”
CSA is discussed at length in any brewing text and a simple Google search will yield countless results. However, Fix lays it out well here:
“Methods for such optimization are covered in, for example, in the references by Bamforth (1999) and Fix (1998). Following C. D. Dalgliesch (1977), it is useful to characterize staling in terms of three basic stages:
• Stage A is the period of stable, “brewery-fresh” flavor.
• Stage B is a transition period in which a multitude of new flavor sensations can be detected.
• Stage C products are the classic flavor tones involved in beer staling.”
He goes on to list an overview of the stages, the highlights of them being:
“Stage A beer is pristine in flavor. During stage B, Dalgliesch described a decline in hop aroma, a decline in hop bitterness,an increase in “ribes aroma” (or sometimes “catty” flavor), and an increase in sweet, toffee-like, or caramel tones. The terms ribes (or currant) and catty are widely used in the United Kingdom and Scandinavia to recall overripe or spoiled fruit or vegetables. Some tasters cite a “black currant” tone (Hardwick, 1978). In truth, these terms describe a wide spectrum of negative flavors developed when beer is in stage B. Toffee or caramel flavors can come from many sources, but those associated with staling will invariably have unattractive cloying notes. These effects are enhanced by residual diacetyl and also by excess heat treatment of wort. Finally, stage C products range from papery or leathery to sherry- or vinegar-like notes.”
While we can agree that we rarely see the last stage of oxidation, varying levels of the characteristic flavors from Stage B are very common, and can be directly attributed to oxidation while kegging, or coming to complete fermentation and racking.
On the Topic of Purity
“Wait a minute, you mean to tell me my 99.9% pure CO2 is not pure enough?” Unknown Homebrewer
Or, the one we always hear regularly, “CO2 is CO2.”
Or when someone asks whether there is a difference between beverage grade CO2 and other types, “Yeah, the price!”
and lastly, “Well if it is so unpure, wouldn’t you oxidize your low oxygen beer by putting it on tap?”
The answer to that last one is actually, YES! It does! It is ultimately the duration between brewery fresh flavors and the onset of staling that we attempt to lengthen. All beer is oxidized or in the process of becoming oxidized, therefore, the question is not whether it is, but rather how long you can prevent it from showing oxidation signs. Here is where you can exercise a degree of control in the matter. We are here today to help you with that, and dispel the persistent rumor that CO2 type doesn’t matter.
Let’s start first by showing industry standard dissolved oxygen levels in the brewery (5):

These are important to us because it gives a frame of reference, and demonstrates that all brewers, even professionals, are plagued by this problem. The lower the number the fresher, better tasting, and more stable the beer will be. If it was only that simple!
Let’s state very clearly at the onset that the even the purest CO2 most homebrewers can get, which is typically 99.9% Grade, contains somewhere between 20-30 ppb of Oxygen (O2) impurity. For our calculations, we are going to assume that homebrewers have access to 99.9% Grade CO2 (1), which typically has a maximum of 30 ppb O2 impurity depending on supplier.
What does this all mean? Homebrewers typically inject their CO2 (as opposed to sparging it (2)) into the finished beer they have made. When you inject gas you are forcing a given weight of gas into the liquid under pressure. All of the carbon dioxide, plus any trace oxygen and nitrogen, gets pushed into the beer and dissolves completely, allowing you to calculate the weight of gas used and extrapolate from there your various gas concentrations (2). So if we assume that our vessels have been purged completely of O2, we know that any O2 impurity in the bottled CO2, gets forced into the beer along with the CO2.
How much exactly? Let’s crunch the numbers.
How Much is TOO Much?
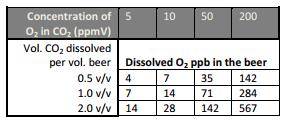
Let’s assume the this scenario: You have procured a bottle of 99.9% Grade CO2. It has a 30 ppb O2 impurity in it. The chart below shows the effect, in ppb O2 dissolved into the finished beer, of various O2 impurities in CO2 (3):
The table shown above was created using information from a 1985 paper on this subject in the MBAA Tech Quarterly (4), written by Nick Huige and his Miller Brewing Company co-workers. While O2 concentrations in the ppb range may not seem very daunting on the surface, Huige’s paper presented damaging evidence of the effect on the finished beer as a result. Let’s try an example:
Where x = the O2 impurity of the CO2 in ppb and y = the volumes of CO2 to be dissolved into the beer.
So for 30 ppb O2 impurity, 18.93 liters of finished beer, and a desired carbonation level of 2.5 volumes, we have:
DO2 = (1.42)*(30)*(2.5) = 106.5 ppb O2 in the carbonated beer
In order to force carbonate to our desired level of CO2 volumes, we would set the regulator at 11.2 psi with a temperature of 38 °F. This would require 58.66 liters of CO2. Yet we also have to account for serving the beer as well. Serving our beer at 12 psi with a temperature of 38 °F would require 39.94 liters of CO2. That is an increase of a factor of 1.47 in required CO2:
DO2 = (106.5)*(1.47) = 156.6 ppb O2 in the carbonated and served beer
So the good news is actually bad news here. We have made a few assumptions, including:
a.) We have assumed you are able to procure, consistently, 99.9% Food Grade CO2 and that the O2 impurity is 30 ppb. Many folks are using 99.5% Grade and that can have O2 impurity up and exceeding 50 ppb.
b.) We assume you have properly purged your keg, including the headspace. Headspace O2 levels alone are enough to be irreparably damaging. Liquid purging with shortened dip tubes and inversion is not as widely practiced as most believe. Most brewers are inadequately purging their kegs.
c.) We assume you have transferred a fully fermented beer in a closed fashion, without picking up any oxygen during the transfer. Again, not everyone is doing this.
So, force carbonating exposes the beer to an inordinate amount of dissolved oxygen versus naturally carbonating over the life of the keg.
Industry cited maximum target O2 content allowed before the onset of accelerated staling in finished beer is 150 ppb. That means before you even serve the beer, force carbonation has aided in exceeding the allowable O2 content into your beer.
So where does that leave us? We have some process improvements and different ways of thinking that may help.
So What Can I do About It?
1.) Stop force carbonating immediately, unless of course you can verify your CO2 source is 99.9% pure (< 10 ppb O2 impurity) or greater. That drops potential oxidation considerably at the onset.
2.) Allow the beer to naturally carbonate itself via spunding. This ensures you are using active yeast as your protection when moving beer. Anytime beer is exposed due to moving or allowing fermentation to end is a potential problem.
3.) Make sure your purging methods are sound. Headspace O2 levels as a result of not fully purging the keg can be irreparably damaging.
4.) Make sure you are practicing closed transfers anytime you move beer, especially beer that has fermented to final gravity.
5.) Only make lagers! Lagers generate natural sulfites, and sulfur compounds which we detail here. I am only kidding (slightly!).
Using these measures is the best safeguard we have to protect that precious beer! Sorry for the grim outlook on oxidation and CO2 but it had to be said.
(1) http://www.cryoservice.co.uk/bulk-liquid-carbon-dioxide.aspx
(2) https://tapintohach.com/tag/o2-in-co2-purity/
(3) https://se.hach.com/asset-get.download.jsa?id=25593625827
(4) Huige, N.J., Charter, W.M.and Wendt, K.W. (1985). pp 92-98: Measurement and control of oxygen in carbon dioxide. Tech. Q. Master Brew. Assoc. Am./TQ-46-3-1985
(5) https://www.hach.com/asset-get.download.jsa?id=50544340479