This will be the last part in the series of blog posts discussing cold side oxygen ingress, and we feel it will be the most thought provoking. We already spoke on purging, CO2 purity, and bottle cap ingress. What about when that keg sits in the serving chamber? All the damage has been done and now it’s safe, right? We know we will get ingress from the CO2, but is that all? Don’t forget you have gaskets in the lid, on the poppets, and on the dip tubes. That’s not all though, as you also have a source of O2 ingress that you probably were not aware of: Your beer lines, both liquid and gas. We imagine that this may create some perplexed and disgusted looks on many people’s faces, “MY WHAT?!?!”
Let’s think about it for a second, and agree that if we know gas laws, especially like with the bottle cap ingress, then it all makes sense. Shown below are some calculations originally presented by Techbrau at the AHA Forum:
“You can calculate the amount of atmospheric O2 that will permeate through the plastic tubing membrane over a given period of time with this equation:
v = P * A * t * (p1 – p0) / d
Where
v = the volume of O2 that diffuses across the membrane
P = the permeability coefficient of the membrane material
A = the area of the membrane
t = time
p1 = the partial pressure of O2 in the atmosphere
p0 = the partial pressure of O2 inside the tubing
d = the thickness of the membraneNon-plasticized PVC tubing has a permeability coefficient of P, where:
P = (0.045 * 10-10cm3 (STP)) * cm/cm2 * s * cm-Hg (1)
Unfortunately, the plasticized version that is required to make flexible tubing has a P that is about 18.75 times higher (2), so our P becomes:
P = (0.84375 * 10-10cm3 (STP)) * cm/cm2 * s * cm-Hg.
A beverage line with 7/16” (1.11125 cm) OD and 3/16” (0.47625 cm) ID gives you a membrane thickness of 4/16” (0.635 cm), which is our d. Let’s assume a 1 m (100 cm) tube length which gives an A of:
A = pi * OD * 100 = 349.109 cm2
A gas line with a 9/16” (1.42875 cm) OD and 5/16” (0.79375 cm) ID also has a 4/16” (0.635 cm) thickness, so we can use the same d = 0.635 cm. The area will be larger:
A = pi * 1.42875 * 100 = 448.855 cm2
Let’s calculate how much O2 ingress we would get per day. There are 24 * 60 * 60 = 86400 seconds in 1 day, so that’s our t.
p1 is the partial pressure of O2 in the atmosphere, which is 0.21 * 76 cm-Hg = 15.96 cm-Hg
p0 can be assumed to be zero. If it wasn’t there wouldn’t be much point to this exercise in the first place.
Let’s plug it in:
Volume of O2 that permeates across 1 meter of beer tubing per day:
v = P * A * t * (p1 – p0) / d = 0.84375 * 10-10 * 349.109 * 86400 * (15.96 – 0) / 0.635 = 0.06396 cm3 (STP)
cm3 (STP) is equivalent to the amount of O2 that would fill one cubic centimeter at standard temperature and pressure. This happens to be 1.43 milligrams worth of O2.
Now let’s do the gas line:
v = P * A * t * (p1 – p0) / d = 0.84375 * 10-10 * 448.855 * 86400 * (15.96 – 0) / 0.635 = 0.08224 cm3 (STP)
Converting the volumes to weights gives us:
0.06396 * 1.43 = 0.09146 milligrams per day through the beer line
0.08224 * 1.43 = 0.11760 milligrams per day through the gas lineBut wait. We only considered a 1 meter long length of beer tubing and a 1 meter long length of gas tubing. Most people use a lot more than that! 15 feet seems to be a standard length of beer tubing. Let’s assume 15 feet worth of gas tubing as well. 15 feet is 4.572 meters, so we have to multiply the numbers we found above by 4.572, giving us:
0.09146 * 4.572 = 0.41815 milligrams per day through the beer line
0.11760 *4.572 = 0.53767 milligrams per day through the gas lineAlright, so that’s a grand total of 0.95582 milligrams of oxygen per day getting into our beer. Assuming we have 18.93 liters of beer sitting in the keg, that’s 0.050492 mg/l = 50.49 ppb per day, or 353.4 ppb per week.
There are better materials out there than plasticized PVC, but all of them will leak gas to a certain degree.”
No need to re-do all the calculations when Tech nails it square on the head there. When it comes to brewing, the final beer is the sum of all its parts. We are going to cite an example of this today, drawing from our other posts in this series, and you will easily be able to see how little things can add up to big things (we always hear folks say, “That’s so insignificant that it just doesn’t apply to me…”).
While kegs our certainly our best defense again O2 intrusion, they are not impervious as they have gaskets; and those gaskets will have ingress as well. So what does a keg allow? For this we can go to the same formula with slightly different coefficients. There isn’t a lot of info on what keg manufacturers use as stock parts for the gaskets, but the popular replacements are Buna-N (nitrile) and silicone (very high permeability) so we will use Buna-N for our examples, as it is conservative. The permeability we found for nitrile is 8.5 * 10-10 cm3 (STP) * cm/cm2 * s * cm-Hg.
v = P * A * t * (p1 – p0) / d
We will assume 18.93 liters of beer.
Lid
P = 8.5 * 10-10 cm3 (STP) * cm/cm2 * s * cm-Hg.
A = 66.88 cm2
t = 1 day
p1 = 15.96 cm-Hg
p0 = 0
d = 0.698 cm
(l = 30.48cm, thickness = 0.69850 cm)
v = P * A * t * (p1 – p0) / d = 8.5 * 10-10 * 66.88 * 86400 * (15.96 – 0) / 0.698 = 0.11230 cm3 (STP)
0.11230 * 1.43 = 0.16058 milligrams per day through Lid Gasket
Post orings
P =8.5 * 10-10 cm3 (STP)· cm/cm2 · s · cm-Hg.
A = 5.74 cm2
t = 1 day
p1 = 15.96 cm-Hg
p0 = 0
d = 0.261 cm
(thickness = 0.261 cm, l = 6.985 cm)
v = P * A * t * (p1 – p0) / d = 8.5 * 10-10 * 5.74 * 86400 * (15.96 – 0) / 0.261 = 0.02577 cm3 (STP) but we have to times that by 2 since we have gas and liquid.
0.02577*2 = 0.05154
0.05154 * 1.43 = 0.07370 milligrams per day through the post o-rings.
Diptube o-rings
P =8.5 * 10-10 cm3 (STP)· cm/cm2 · s · cm-Hg.
A = 3.16 cm2
t = 1 day
p1 = 15.96 cm-Hg
p0 = 0
d = 0.3175 cm
(thickness = 0.3175 cm, l = 3.175 cm)
v = P * A * t * (p1 – p0) / d = 8.5 * 10-10 * 3.16 * 86400 * (15.96 – 0) / 0.3175 = 0.01160 cm3 (STP) but we have to multiply that by 2 since we have gas and liquid.
0.01160 * 2 = 0.0232
0.0232 * 1.43 = 0.03317 milligrams per day through the diptube o-rings.
So combined they let in 0.26765 milligrams per day. Again assuming we have 18.93 liters of beer sitting in the keg, that’s 0.01413 mg/l = 14.13 ppb per day, or ~98.9725 ppb per week.
So at this point, let’s also include numbers previously calculated in our other cold-side oxidation posts:
Standard Homebrew practices:
Purging: Using a “standard” homebrew purge of 5 cycles leaves you with roughly 10,000ppb in the head space so depending how much headspace you leave, will net you roughly 1-4 ppm (yes, parts per million!) of dissolved O2 (verified with a DO meter). Since we are all about conservative here we will choose 2 ppm, which already puts you way over the limit, but we digress.
So, 2 ppm added here.
CO2 Purity: Using a “standard” bottle of 99.9% Grade CO2. It has a 30 ppm O2 impurity in it.
So for 30 ppm O2 impurity, 18.93 liters of finished beer, and a desired carbonation level of 2.5 volumes, we have:
DO2 = (1.42) * (30) * (2.5) = 106.5 ppb O2 in the carbonated beer
In order to force carbonate to our desired level of CO2 volumes, we would set the regulator at 11.2 psi with a temperature of 38 °F. This would require 58.66 liters of CO2. Yet we also have to account for serving the beer as well. Serving our beer at 12 psi with a temperature of 38 °F would require 39.94 liters of CO2. That is an increase of a factor of 1.47 in required CO2:
DO2 = (106.5) * (1.47) = 156.6 ppb O2 in the carbonated and served beer
156.6 pbb added here, 2.0156 ppm total now.
Beverage lines: Using “standard” serving, and gas lines you are looking at 50.49 ppb per day over the entire life of the keg. If your kegs last 1 month you are looking at 1.338 ppm.
1.338ppm added here, 3.35ppm total now.
Keg gaskets: Lastly, using “standard” keg gaskets and o-rings you are looking at 14.13 ppb per day, and 423.9 ppb for the month.
423.9 ppb added here, and for a grand total of 3.77 ppm total over the life of that beer! Using best case scenarios, this is still a VERY bleak outcome.
Now lets contrast using the “sum of all parts” approach we firmly believe in here. We will ONLY outline the oxidation outcomes here, as we already know of the countless other benefits to these methods.
Modern Brewhouse Brewing Practices:
Purging: Using the fermenter purging method you get the keg down to at most 5 ppb.
5 ppb added here
Spunding: We use spunding to carbonate and purge vessels for us. So that 5 ppb we were left with in the keg, and the gasket/oring ingress we see in the keg is getting actively consumed until the beer stops fermenting. For all intents and purposes we are now back to zero again.
All DO gone back at 0 ppm.
CO2 Purity: We are stuck here like everyone else. So we will be using a “standard” bottle of 99.9% Grade CO2. It has a 30 ppm O2 impurity in it. We are, however, only using it for serving our beer. At 12 psi with a temperature of 38 °F, this would require 39.94 liters of CO2. We are looking at 50.1 ppb of O2 in the served beer.
50.1 ppb added here, 50.1 ppb total now.
Beverage lines: We are going to use “special” lines that have added barriers in them, and on top of that we are disconnecting both liquid and gas when not serving. So we only add O2 ingress here when we are actually serving. Let’s figure that we take 5 minutes to serve a beer. In a full keg there are ~40 16 fl. oz. servings, so let’s assume 5 * 40 = 200 minutes (12000 seconds) of ingress. 6.63 ppb added here.
6.63 ppb added here, 56.73 ppb total now.
Keg gaskets: We will be using “standard” keg gaskets and o-rings as well, however, since we are disconnecting when not in use, we only have to factor our 200 minutes (12000 seconds) of ingress for the post o-rings. So for post o-rings we will round up to 1 ppb and we are looking at 14.13 ppb per day, and 423.9 ppb for the month.
423.9 ppb added here, and for a grand total of 480.63 ppb total over the life of that beer. Using these practices we shaved off 3.28 ppm! of O2, a reduction of nearly 87%! Remember when you hear people say that all those “little” parts don’t matter? They always add up! Beer is the sum total of ALL it’s parts.
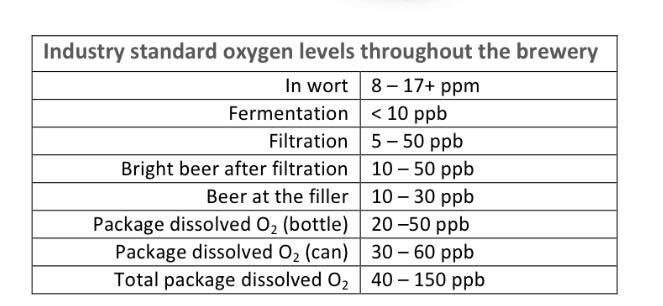
It seems monotonous at this point but we have to post the Industry standard levels for reference.
So what can we do?
The easy answer is to disconnect your lines when not serving. The re-connection procedure would then be to purge the CO2 lines, pour and drain beer left in the liquid lines, then fill. However, as an aside, say you are like many folks and have a CO2 manifold, any tubing up to the manifold and after it will have oxygen in it. You need to make sure and purge all lines and connections to get rid of all O2 in the lines before re-attaching. Then lightly press the QD on the post so that CO2 floods the area for a second or two then attach.
You can also get better tubing.
For liquid:
Bevlex® Series 235 Polyefin Tube with Internal Dual Barrier is a great tubing as it has another liner in it. I have ran it for about 10 years now with great success.
For gas:
Bev-Seal® Series 176 “Proprietary Barrier Protected core tube design resists permeation of the CO2 gas, while at the same time protecting the gas from ingression of external contaminants.” I have ran this as well for some time with great results.
Really, as we have said already, all beer is oxidized and we are in a race against time. The best practices for brewery fresh (stage A) beer is to: Make Clear wort, have a healthy/strong/quick fermentation with active yeast, spund to carbonate, get the beer cold immediately after fermentation is completed (once fermentation is complete, staling starts), and NEVER allow the beer to get warm. Source CO2 from a known good source. Once tapped, disconnect lines when not in use (make sure and purge when connecting), and lastly drink quickly! Those are the best things we can do.
At the end of the day, what we tried to do here with this series is not to promote the “Doom and Gloom” view on cold-side oxidation, but rather show folks what really scientifically happens. Just because you don’t think oxygen can permeate lines and gaskets does not will the O2 to stop doing it. These are proven gas laws. So maybe try some (or all) see if it yields fresher beer for you!
(1) http://www.faybutler.com/pdf_files/HowHoseMaterialsAffectGas3.pdf
(2) http://www.tappi.org/content/events/07place/papers/fischer.pdf
(3) https://imageserv5.team-logic.com/mediaLibrary/99/D116_20Haibing_20Zhang_20et_20al.pdf